India’s own CAR-T cell therapy
03-11-2023
08:38 AM
1 min read

What’s in today’s article?
- Why in news?
- CAR-T cell therapy
- What is CAR-T cell therapy, and how do CAR-T cells find and destroy cancer cells?
- How effective and different is this from other cancer treatments like, say, chemotherapy?
- NexCAR19
- What is NexCAR19?
- Who can get the NexCAR19 therapy?
- Are children eligible for the therapy too?
- What are the side-effects of this therapy?
- How much will this treatment cost?

Why in news?
- NexCAR19 is India’s first indigenously-developed CAR-T cell therapy.
- Recently, the Central Drugs Standard Control Organisation (CDSCO) granted market authorisation for NexCAR19 to ImmunoACT, a company incubated by IIT Bombay.
- This paves the way for the commercial launch of this therapy in India, where it is expected to be available to cancer patients at a tenth of the cost abroad.
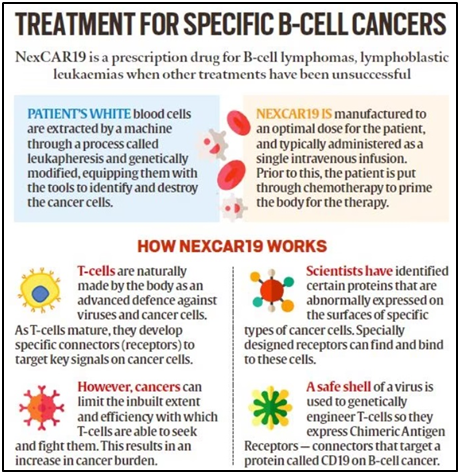
What is CAR-T cell therapy, and how do CAR-T cells find and destroy cancer cells?
- CAR-T is a revolutionary therapy that modifies immune cells, specifically T-cells, by turning them into potent cancer fighters known as CAR-T cells.
- T-cells are special cells (white blood cells that find and fight illness and infection) whose primary function is cytotoxic, meaning it can kill other cells.
- CAR-T therapy, we genetically modify them into cancer-fighting cells.
- These supercharged cells are then put back into the body, and they go after cancer cells — especially in blood cancers like leukaemia and lymphomas.
How effective and different is this from other cancer treatments like, say, chemotherapy?
- While chemotherapy and immunotherapy may add a few months or years to a cancer patient’s life, cell-and-gene therapy is designed to cure and provide lifelong benefit.
- It makes treatment easier with a one-time therapy [unlike several sessions of chemotherapy].
- It is a lifeline for non-responsive cancer patients.
What is NexCAR19?
- NexCar19 is a type of CAR-T and gene therapy developed indigenously in India by ImmunoACT, which is a company incubated at IIT Bombay.
- This therapy is designed to target cancer cells that carry the CD19 protein.
- This protein acts like a flag on cancer cells, which allows CAR-T cells to recognise and attach themselves to the cancer cells and start the process of elimination.
- India is now one of the first developing countries to have its indigenous CAR-T and gene therapy platform.
Who can get the NexCAR19 therapy?
- The therapy is for people with B-cell lymphomas who didnot respond to standard treatments like chemotherapy, leading to relapse or recurrence of the cancer.
- B-cell lymphoma is a type of cancer that starts in white blood cells called lymphocytes.
- Lymphocytes make antibodies, which are proteins that help fight infections.
- They are often found in lymph nodes or other lymphoid tissues such as the spleen.
- Essentially, patients only need to give a blood sample at their clinic, and come back in 7-10 days for reinfusion.
- The blood goes to the lab, where the T-cells are genetically modified. In a week to 10 days, these cells return to the clinic for patient reinfusion.
- Recovery typically occurs within two weeks after one cycle of the treatment.
Are children eligible for the therapy too?
- For now, ImmunoACT has received CDSCO approval for use in patients aged 15 years and older.
- The paediatric trial phase is currently underway at the Tata Memorial Hospital, in collaboration with IIT-Bombay.
What are the side-effects of this therapy?
- NexCar19leads to significantly lower drug-related toxicities. It causes minimal damage to neurons and the central nervous system, a condition known as neurotoxicit
- Neurotoxicity can sometimes occur when CAR-T cells recognise the CD19 protein and enter the brain, potentially leading to life-threatening situations.
- The therapy also results in minimal Cytokine Release Syndrome (CRS).
- CRS is characterised by inflammation and hyperinflammation in the body due to the death of a significant number of tumour cells, as CAR-T cells are designed to target and eliminate cancer cells.
How much will this treatment cost?
- Currently, the treatment at a price range of Rs 30-40 lakh and the target to the company is to bring it down to Rs 10-20 lakh.
- As technology matures and manufacturing processes improve, it is anticipated that the cost will decrease.
Q1) What are Lymphocytes?
Lymphocytes are a type of white blood cell that are part of the immune system.
Q2) What is ImmunoACT?
ImmunoACT is a company that develops cell and gene therapies in India. The company was spun out of IIT-Bombay in 2018. ImmunoACT's mission is to provide affordable access to novel autologous CAR-T cell therapies.
Source: India’s own CAR-T cell therapy: What is it, when will it be available, and at what cost? | Down To Earth | Times of India