National Medical Commission
26-08-2023
01:29 PM
1 min read

What’s in Today’s Article?
- Why in News?
- About National Medical Commission
- Medical Practitioner (Professional Conduct) Regulations, 2023
- Issued Raised by the Indian Medical Association (IMA)
- News Summary
Why in News?
- The National Medical Commission (NMC) has put on hold the regulations that make it mandatory for doctors to prescribe generic drugs.

About National Medical Commission:
- The National Medical Commission is a statutory body established under the National Medical Commission Act, 2019.
- The NMC replaced the erstwhile Medical Council of India (MCI) which was established in 1934.
- Objectives of NMC –
- Improve access to quality and affordable medical education;
- Ensure availability of adequate and high-quality medical professionals in all parts of the country;
- Promote equitable and universal healthcare that encourages community health perspective and makes services of medical professionals accessible to all the citizens;
- Encourages medical professionals to adopt latest medical research in their work and to contribute to research;
- Objectively assess medical institutions periodically in a transparent manner;
- Maintain a medical register for India;
- Enforce high ethical standards in all aspects of medical services;
- Have an effective grievance redressal mechanism.
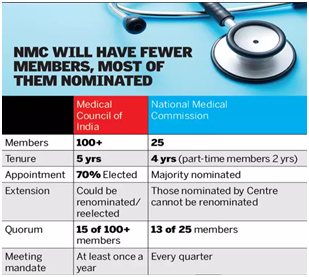
- Composition of NMC –
-
- NMC is a 25-member body, majority of them being nominated by the Central government.
- Tenure of NMC members is four years (except for part-time members whose tenure is two years).
- The NMC has 11 part-time members representing states or state medical councils.
- The NMC chairpersons and other members, nominated by the Central government, cannot be renominated.
- Any decision requires approval of the majority (minimum 13 out of 25) of the Commission.
-
Medical Practitioner (Professional Conduct) Regulations, 2023:
- On August 2nd, the National Medical Commission had published the Medical Practitioner (Professional Conduct) Regulations, 2023 aimed at reshaping prescription practices.
- It mandated that registered medical practitioners prescribe medications using “generic”, “non-proprietary”, or “pharmacological” names.
- The guidelines define a generic drug as a “drug product that is comparable to brand/reference listed product in dosage form, strength, route of administration, quality and performance characteristics, and intended use.”
- It says branded generic drug is one which has come off patent and is manufactured by drug companies and sold under different companies’ brand names.
- The guidelines say, “Every RMP (Registered Medical Practitioner) should prescribe drugs using generic names written legibly and prescribe drugs rationally, avoiding unnecessary medications and irrational fixed-dose combination tablets.”
- The guidelines have also talked about punitive measures against those violating the directive.
- Besides the instructions on generic drugs, the NMC guidelines included directives on issues ranging from continued medical education, usage of social media platforms and maintaining a dynamic register of doctors.
- It also barred doctors from attending events sponsored by pharmaceutical companies.
- However, the NMC guidelines have not gone down well with the Indian Medical Association (IMA).
Issued Raised by the Indian Medical Association (IMA):
- The IMA issued a statement in response to the regulations introduced by the NMC.
- The IMA says the biggest impediment to generic drugs is the uncertainty about its quality.
- IMA said that the quality control in the nation being very weak, there’s practically no guarantee of the quality of drugs and prescribing drugs without assured quality would be detrimental to patient health.
- The statement added that less than 0.1% of the drugs manufactured in India are tested for quality.
- The IMA said that step should be deferred till the Government can assure the quality of all the drugs released into the market.
- The statement says patient care and safety are not negotiable.
- The IMA says it has been demanding for long that only good quality drugs should be made available in the country and prices should be uniform and affordable.
- It urges the Government to have ‘one drug, one quality, one price’ system whereby all brands should either be sold at the same price or banned and only generics allowed while ensuring highest quality of these drugs.
News Summary:
- In light of the criticism received by the Indian Medical Association (IMA) as well as the as the Indian Pharmaceutical Alliance (IPA), the National Medical Commission put on hold the Medical Practitioner (Professional Conduct) Regulations, 2023.
- Even the country’s apex drug regulator, the Central Drugs Standard Drug Control Organisation (CDSCO), questioned the language in the notification.
- The participating bodies suggested that the guidelines be kept in abeyance until the WHO’s good manufacturing practices are implemented.
- The participants said that prescribing only generic drugs will prompt pharmacies to sell generic drugs at high-profit margins, disincentivising firms that manufacture quality branded generics.
Q1) What is the size of healthcare industry in India?
India's overall healthcare industry is estimated to be around $372 billion in 2022, with a compound annual growth rate (CAGR) of 22 percent.06-Apr-2023
Q2) What is meant by medical tourism?
Medical tourism (also called medical travel, health tourism or global healthcare) is a term used to describe the rapidly-growing practice of travelling across international borders to seek healthcare services.
Source: NMC puts on hold rules for doctors to prescribe only generic medicines | ToI