Patent Monopoly/Evergreening: India rejects Johnson & Johnson’s attempt to extend monopoly on lifesaving TB drug
26-08-2023
12:13 PM
1 min read

What’s in today’s article?
- Why in News?
- What is the Evergreening of Patents?
- Why is Patent Monopoly/Evergreening Granted?
- What is the Supreme Court’s Verdict in this Regard?
- News Summary Regarding Indian Patent Office’s Recent Decision
Why in News?
- The Indian Patent Office rejected U.S. pharmaceutical giant Johnson & Johnson’s (J&J) attempt to extend its monopoly on the manufacturing of the anti-tuberculosis drug Bedaquiline in India beyond July 2023.
- This will pave the way for generic drug manufacturers (Lupin and Macleods) to produce Bedaquiline tablets, thus ensuring cheaper (currently priced at $400 per six-month course) and wider access to the drug.

What is the Evergreening of Patents?
- The evergreening of patents is a practice of tweaking drugs in order to extend their patent term and thus their profitability.
- The Indian Patents Act 1970 introduced many provisions [Sections 3(d), 53(4) and 107A] to prevent the mischievous practice of “evergreening” of patents.
- This is to aid millions of people who can't afford the expensive modified drugs, as well as the development of the domestic generic drug market.
- However, evergreening patents on drugs (diabetes, cancers, cardiovascular diseases, etc) continue to be granted to pharmaceutical innovator companies by the Indian Patent Office and enforced through courts.
Why is Patent Monopoly/Evergreening Granted?
- The economic assumption behind the Patent Bargain (private risk is rewarded and incentivised in return for a limited private monopoly right) is to have a trickle-down impact that benefits the general population.
- Therefore, patent monopolies are granted to innovators in the hope that they disclose something new, inventive and of industrial value to the public.
- However, the Patent Bargain becomes a Faustian bargain (in which a person abandons his or her moral principles to obtain benefits),
- Undermining competition in the market and
- Enables patentees to extract more from the society than permitted.
What is the Supreme Court’s Verdict in this Regard?
- In Novartis AG v. Union of India & Others (2013), the apex court held that the legislative intent is to prevent evergreening of a patent monopoly that in no way enhances the drug’s therapeutic efficacy.
- However, the SC’s verdict has not yielded any positive outcomes both from the Patent Office and subordinate courts, rather it delayed entry of generic versions, adversely affecting the availability of affordable medicines.
News Summary Regarding Indian Patent Office’s Recent Decision:
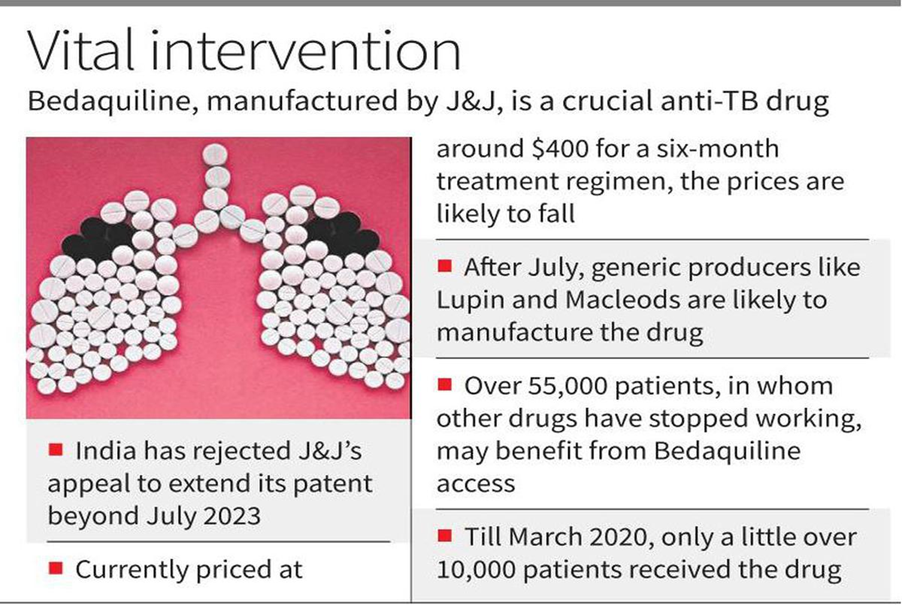
Image Caption: India rejects evergreening of Bedaquiline
- Bedaquiline is a crucial drug in the treatment of multi-drug resistant TB patients for whom the first-line drug treatment (using Isoniazid, Rifampicin, Pyrazinamide and Ethambutol) has stopped working.
- According to the latest estimates, over 55,000 patients who had developed multi-drug resistant TB could have benefited from access to Bedaquiline in 2019.
- Therefore, it is high time that alternate manufacturers start supplying Bedaquiline at lower prices, especially as TB programmes around the world plan to scale-up the all-oral, shorter, six-month drug-resistant TB regimen.
- Since 2007, J&J has indulged in ‘evergreening’ by making multiple claims in its applications for patent extensions.
- When the company filed for evergreening of its patent on fumarate salt (a formulation of Bedaquiline), the practice was challenged by two TB survivors in 2019.
- The Indian Patent Office, in the order passed, stated that the invention claimed was obvious and does not involve any inventive step (under Section 3(e) of the Patents Act), and is therefore non-patentable.
- Section 3(d) of the Patents Act states that salt forms and derivatives of known substances are not patentable.
- Hence, the applicant cannot claim a patent on these methods and compositions of salt forms that have been known in the scientific world for more than three decades.
- However, J&J will continue to hold the patent on Bedaquiline in other major markets such as South Africa, meaning that Indian generic manufacturers will be unable to export the drug there.
Q1) What is multi-drug resistant TB?
Multidrug-resistant TB is caused by TB bacteria that are resistant to at least isoniazid and rifampin, the two most potent TB drugs. Such patients are treated with Bedaquiline tablets.
Q2) What is Section 3(d) of the Indian Patents Act?
It bars the patenting of a “mere use of a known method” unless such known method results in a new product or employs at least one new reactant.
Source: India rejects Johnson & Johnson’s attempt to extend monopoly on lifesaving TB drug
