Why Does India Not Have a Law to Recall Bad Drugs From the Market?
26-08-2023
12:29 PM
1 min read

What’s in today’s article?
- Background (Context)
- Delay in Legalising the Recalling of Substandard Drugs
- What Are the Consequences of This Delay?
- How Can This Situation be Improved Upon?
- About Drug Controller General of India (DCGI)
Background (Context)
- Recently, Abbot, a multinational pharmaceutical company, published a public notice in newspapers alerting people about a mislabelled batch of medicine that it had inadvertently shipped to the market.
- Such recalls take place regularly in the U.S., including by Indian companies.
- This is because, in the U.S., pharmaceutical companies are required to recall from the market those batches of drugs that have failed to meet quality parameters.
- India, on the other hand, has been mulling the creation of a mandatory recall law for substandard drugs since 1976.
- However, till date, no such no law exists that mandates such medicine be removed from the market to this day.

Delay in Legalising the Recalling of Substandard Drugs
- In 1976, the Drugs Consultative Committee (DCC), and the Central Drug Standard Control Organisation (CDSCO), discussed the issue of drug recalls.
- DCC consists of all the state drug controllers along with senior bureaucrats from the Ministry of Health.
- The minutes of this meeting record a discussion on how drugs ordered to be recalled by a state drug controller in one state were found to be on sale in a neighbouring state.
- The meeting resolved to have greater cooperation between various state drug controllers in order to facilitate better coordination to recall and destroy drugs that failed tests.
- But this decision never translated into amending the law to create a binding legal structure to enforce such recalls.
- In 2012, the CDSCO proposed a set of draft recall guidelines. However, only the Ministry of Health or, more importantly, the Drug Regulation Section of the Ministry, can initiate the process to make binding rules or legislation.
- The same issue came up again in 2018 and 2019 at the meetings of the DCC, but India still lacks a recall law, 46 years on.
What Are the Consequences of This Delay?
- Every month, dozens of drugs fail random-testing in government laboratories.
- People, including children, are almost certainly dying or suffering from adverse health events because substandard drugs are not swiftly removed from the market.
How Can This Situation be Improved Upon?
- Currently, India’s Drug Regulatory Framework is highly fragmented, with each state having its own drug regulator.
- This has led to inconsistent enforcement of the law and jurisdictional issues because drugs from one state can be sold in another but drug inspectors from one state cannot inspect manufacturing facilities in other states.
- To create an effective recall mechanism, the responsibility of recalling drugs has to be centralised.
- There has to be a single authority that has the legal power to hold companies liable for failures to recall drugs from across the country, and further, to also search and seize batches of failed medicine.
- All manufacturing facilities should be licensed by a national regulator.
About Drug Controller General of India (DCGI)
- Drugs Controller General of India is the head of department of the Central Drugs Standard Control Organization (CDSCO).
- The CDSCO is the Central Drug Authority for discharging functions assigned to the Central Government under the Drugs and Cosmetics Act, 1940.
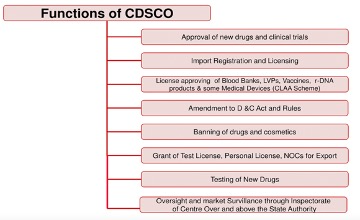
Image Caption: Functions of CDSCO
- Nodal Ministry – Ministry of Health & Family Welfare
Q1) What is the Red Line Campaign in Pharma?
Tablet packs with a red stripe indicate that these cannot be purchased without a doctor's prescription.
Q2) What are the 8 diseases covered under Mission Indradhanush?
Tuberculosis, Diphtheria, Pertussis, Tetanus, Polio, Hepatitis B, Pneumonia and Meningitis due to Haemophilus Influenzae type b (Hib), Measles.
Source: Explained | Why does India not have a law to recall bad drugs from the market?
