Proton Emission Latest News
An international collaboration of researchers recently detected and measured the half-life of the heaviest proton emitter, the 188At (astatine) isotope, which decayed by emitting a proton.
About Proton Emission
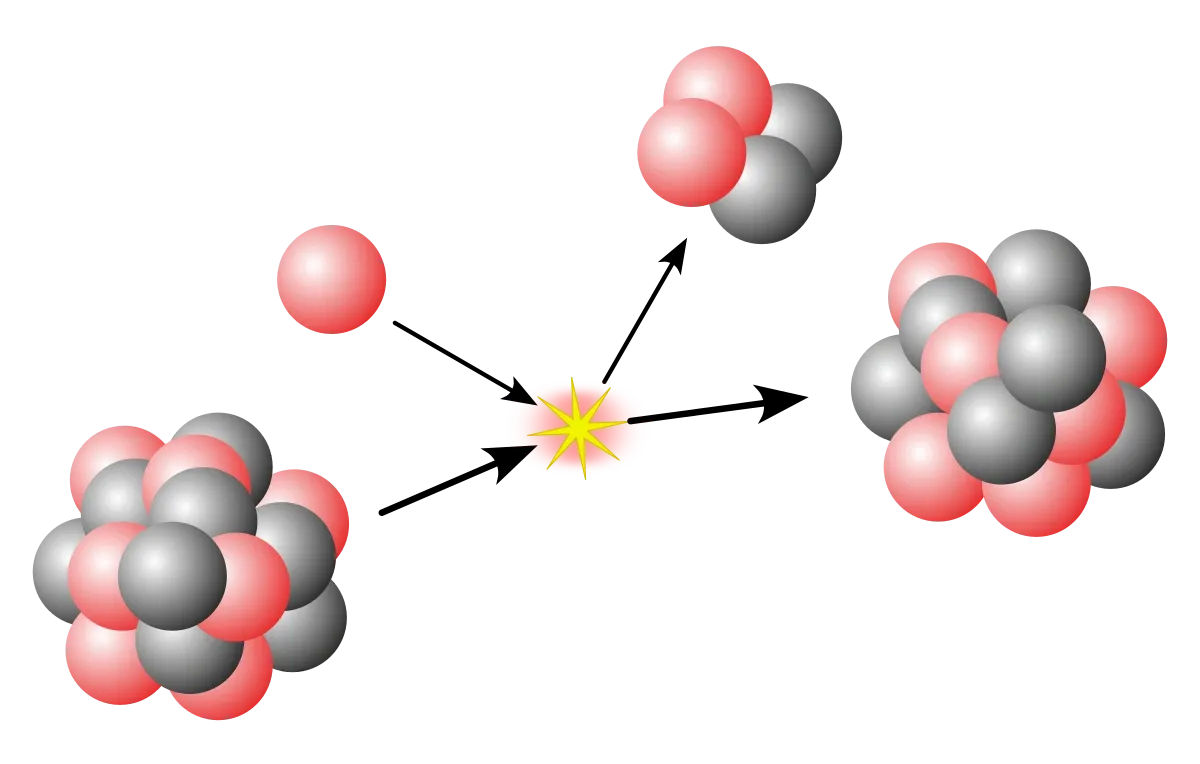
- Proton emission (also known as proton radioactivity) is a rare form of radioactive decay in which a proton is ejected from a nucleus.
- Radioactive decay is the process of an unstable atom transitioning to a more stable form.
- It may do so by releasing subatomic particles and energy or by capturing an orbital electron into the nucleus and releasing energy.
- Proton emission can occur from high-lying excited states in a nucleus following a beta decay, in which case the process is known as beta-delayed proton emission, or can occur from the ground state (or a low-lying isomer) of very proton-rich nuclei, in which case the process is very similar to alpha decay.
- Alpha decay is a type of radioactive decay in which an unstable nucleus emits an alpha particle. An alpha particle consists of 2 protons and 2 neutrons, which is the same as a helium nucleus (⁴₂He).
- Beta decay occurs when a nucleus emits a beta particle, which can be an electron (β⁻) or a positron (β⁺).
- For a proton to escape a nucleus, the proton separation energy must be negative - the proton is therefore unbound, and tunnels out of the nucleus in a finite time.
- Proton emission is not seen in naturally-occurring isotopes; proton emitters can be produced via nuclear reactions, usually utilising some kind of particle accelerator.
- The rate of proton emission is governed by the nuclear, Coulomb, and centrifugal potentials of the nucleus, where centrifugal potential affects a large part of the rate of proton emission.
- The half-life of a nucleus with respect to proton emission is affected by the proton energy and its orbital angular momentum.
- Half-life is the time that it takes for half of the original value of some amount of a radioactive element to decay.
Proton Emission FAQs
Q1. What is proton emission?
Ans. It is a rare form of radioactive decay in which a proton is ejected from a nucleus.
Q2. In which condition does proton emission typically occur?
Ans. In proton-rich nuclei.
Q3. What is required for a proton to be emitted from the nucleus?
Ans. Negative proton separation energy.
Source: TH