Why in News?
- USA’s Victoria Gray is the world’s first sickle cell anaemia (SCA) patient to recover with the revolutionary gene-editing therapy.
- Gray underwent a clinical trial in 2017 for the drug Casgevy, which uses the innovative gene-editing tool CRISPR-Cas9 (Clustered Regularly Interspaced Short Palindromic Repeats and associated protein 9).
What’s in Today’s Article?
- Why in News?
- What is Sickle Cell Anaemia (SCA)?
- How the Pathbreaking Gene Therapy for SCA Works?
- Significance of this Pathbreaking Gene Therapy for SCA for India
- Concerns Regarding the Gene Therapy for SCA and Way Ahead
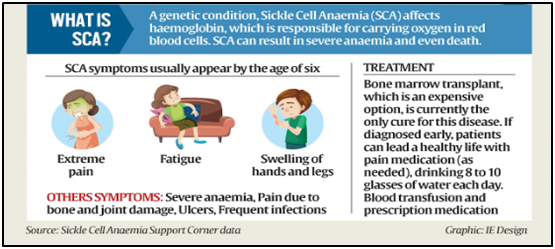
What is Sickle Cell Anaemia (SCA)?
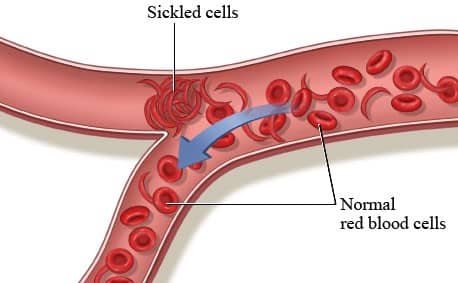
- A genetic disease, SCA arises from mutations in haemoglobin-carrying genes, causing red blood cells to assume a crescent shape.
- This potentially obstructs blood flow and leads to severe pain, organ damage, strokes, and other complications.
- Globally, the current remedy for the disease is limited to bone marrow transplants (which must come from a closely matched donor and carries a risk of rejection).
How the Pathbreaking Gene Therapy for SCA Works?
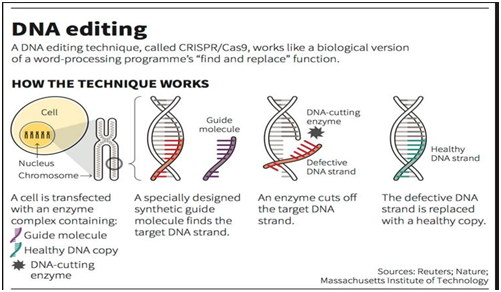
- The CRISPR-Cas9 technique basically involves modifying the patient’s DNA, specifically targeting and replacing the faulty haemoglobin gene with a healthy one.
- To do this, stem cells are taken out of the bone marrow, edited in a laboratory and then infused back into the patient.
- This restores normal haemoglobin function, offering a potential cure for a lifetime.
- About 29 patients were administered the treatment (along with Gray), of whom 28 have no pain and will be followed up for lasting cure.
- The therapy also works for patients suffering from transfusion-dependent ß-thalassemia.
- Recently, the UK approved (became first country to do so)the therapy – called Casgevy, as it has been the only permanent, innovative and first-of-its-kind gene-editing treatment option.
Significance of this Pathbreaking Gene Therapy for SCA for India
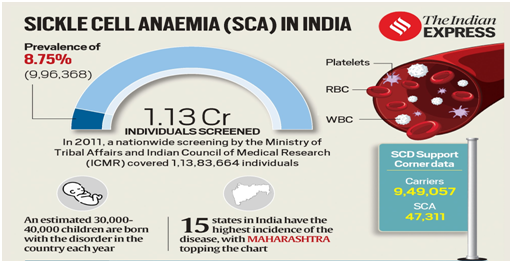
- The breakthrough is of particular significance to India, which has the second-highest disease burden of SCA globally after African countries.
- An estimated 30,000-40,000 children in India are born with the disorder every year.
- In 2019, a nationwide screening by the Ministry of Tribal Affairs and the Indian Council of Medical Research (ICMR) found the disease to be prevalent in 8.75% of those screened.
- Latest data shows that one in 86 births among the Scheduled Tribe (ST) population is affected by SCA, with higher rates in central, western and southern India.
- In her 2023-24 Budget presentation, the Finance Minister of India had said that the government aimed to eliminate the disease by 2047.
Concerns Regarding the Gene Therapy for SCA and Way Ahead
- While Vertex and CRISPR Therapeutics (the manufacturers) have yet to set a price for the gene-editing therapy in the UK, at the moment it is expected to exceed Rs 1 crore in India, which could potentially limit access.
- However, government intervention and subsidies, along with crowdfunding and philanthropy, can enable access to cure.
- The UK’s approval would inspire Indian researchers to develop their own innovative therapies of CRISPR like they did with CAR-T for blood cancer, ultimately making the treatment more affordable.
Q1) What are genetic disorders?
Genetic disorders are a category of diseases that includes certain types of birth defects, chronic diseases, developmental problems, and sensory deficits that are inherited from one or both parents.
Q2) Why is sickle cell anaemia common in tribes of India?
The tribal areas in India were endemic to malaria for many years leading to an evolutionary trait – their red blood cells were becoming sickle-shaped. This led to their high susceptibility to sickle cell disease, including alpha-thalassemia.
Source: UK approves gene therapy for sickle cell anaemia. Why it offers hope for Indian patients
Last updated on March, 2026
→ UPSC Final Result 2025 is now out.
→ UPSC has released UPSC Toppers List 2025 with the Civil Services final result on its official website.
→ Anuj Agnihotri secured AIR 1 in the UPSC Civil Services Examination 2025.
→ UPSC Marksheet 2025 Will be out soon.
→ UPSC Notification 2026 & UPSC IFoS Notification 2026 is now out on the official website at upsconline.nic.in.
→ UPSC Calendar 2026 has been released.
→ Check out the latest UPSC Syllabus 2026 here.
→ UPSC Prelims 2026 will be conducted on 24th May, 2026 & UPSC Mains 2026 will be conducted on 21st August 2026.
→ The UPSC Selection Process is of 3 stages-Prelims, Mains and Interview.
→ Prepare effectively with Vajiram & Ravi’s UPSC Prelims Test Series 2026 featuring full-length mock tests, detailed solutions, and performance analysis.
→ Enroll in Vajiram & Ravi’s UPSC Mains Test Series 2026 for structured answer writing practice, expert evaluation, and exam-oriented feedback.
→ Join Vajiram & Ravi’s Best UPSC Mentorship Program for personalized guidance, strategy planning, and one-to-one support from experienced mentors.
→ Shakti Dubey secures AIR 1 in UPSC CSE Exam 2024.
→ Also check Best UPSC Coaching in India