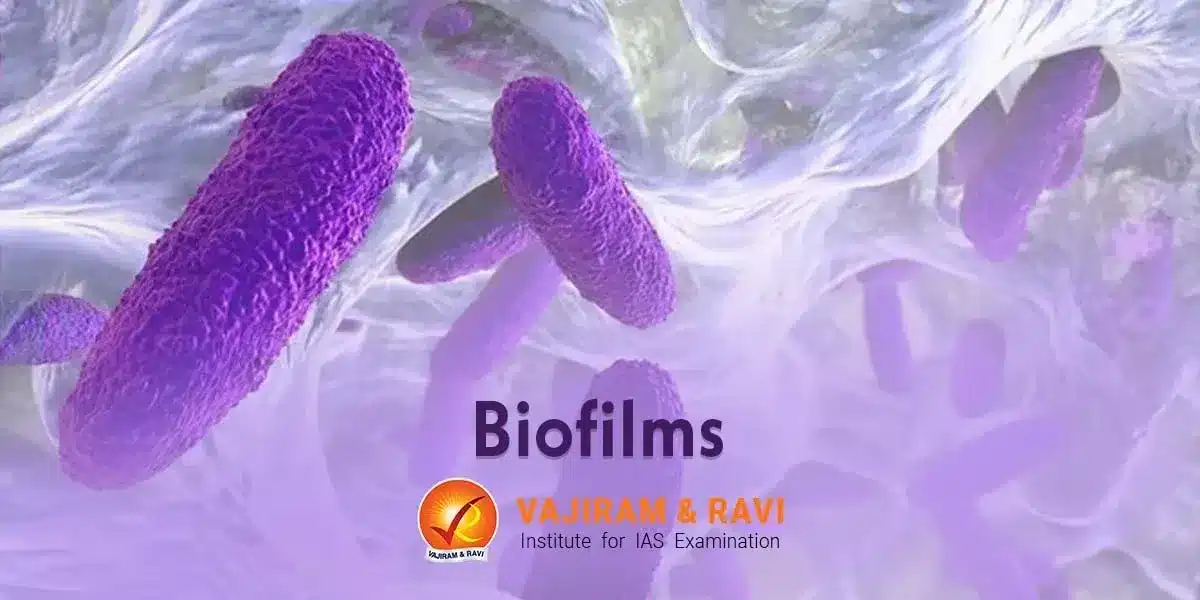
Biofilms are a group of one or more types of microorganisms capable of developing on various surfaces. The microorganisms that can form biofilms include bacteria, fungi, and protists. They are held together by sugary molecular strands, collectively termed “extracellular polymeric substances,” allowing them to develop complex, three-dimensional, attached communities that are resistant to attacks that would destroy individual cells not part of a biofilm colony. They pose a challenge to eradicate and are the cause of many persistent infections and industrial fouling issues.
About Biofilms
A biofilm is an aggregation of microorganisms such as bacteria, fungi, protozoa etc. that grow on a surface and are enclosed in a self-produced extracellular matrix. The biofilm matrix is composed of extracellular polymeric substances (EPS) which are secreted by the microorganisms and provide structural integrity and protection. Some key features:
- Biofilms can form on both biotic and abiotic surfaces.
- Common surfaces include teeth, bones, implants, ship hulls, pipelines, rocks etc.
- Cells are attached to the surface, but also to each other through the EPS matrix. This matrix acts like a glue holding the biofilm together.
- The EPS matrix is made up of polysaccharides, proteins, nucleic acids and lipids.
- It provides structural stability and protection against environmental stresses.
- They display an altered phenotype compared to their free-floating or planktonic counterparts. They show increased resistance to antibiotics, disinfectants and environmental stresses.
- Biofilms can contain single species or multiple species living synergistically. The aggregation provides advantages like enhanced access to nutrients and collective resistance mechanisms.
- Biofilms form through a developmental process involving initial attachment, maturation, dispersal and re-attachment. Cell signalling mechanisms like quorum sensing regulate their development.
Biofilm Formation
Its formation occurs through a series of steps that involve complex biochemical signals and interactions between the microorganisms.
- Initial attachment: Free-floating microorganisms come into contact with a surface and attach.
- Irreversible attachment: The microorganisms begin to produce extracellular polymeric substances (EPS) and firmly attach to the surface. The production of EPS makes this attachment irreversible.
- Early development of biofilm architecture: The microorganisms multiply and form a layer known as a microcolony. The EPS matrix develops further during this stage.
- Maturation: It continues to develop complexity and thickness. Water channels form within the biofilm to allow nutrients and waste to circulate.
- Dispersion: Portions of the biofilm disperse to colonise other areas. This can occur due to environmental triggers or when the biofilm reaches a certain stage of maturity.

Functions of Biofilms
It serves several functions that promote the growth and survival of the microorganisms within them:
- Protection: The EPS matrix provides a physical barrier against environmental threats like desiccation, UV exposure, pH changes, salinity, metal toxicity and predation.
- Nutrient access: The biofilm structure with water channels allows efficient circulation of nutrients to cells deep within the matrix.
- Cellular communication: The proximity of the cells within a biofilm allows for ease of cellular communication through signal molecules.
- This can enhance the survival and growth of the microorganisms.
- Collective antimicrobial resistance: It displays up to 1000 times greater antibiotic resistance than planktonic cells.
- The EPS matrix limits antibiotic penetration. Also, slow growth in biofilms contributes to resistance.
- Gene transfer: High cell density and EPS matrix aids horizontal gene transfer through plasmids and transposons.
- This facilitates the acquisition of new traits like antimicrobial resistance.
Biofilms in Disease
Many recalcitrant bacterial infections are attributed to biofilms. Its formation is a key virulence factor for many pathogens.
- Dental plaque: Plaque is a classic example of a natural multispecies biofilm. It is responsible for dental cavities and periodontal disease.
- Urinary tract infections (UTI): The most common cause of catheter-associated UTIs is biofilm formation by Uropathogens like E. colion the catheter surface This leads to chronic and resistant infections.
- Cystic fibrosis pneumonia: Chronic lung infections due to biofilm formation by Pseudomonas aeruginosa and Burkholderia cepacia complex are the primary cause of morbidity and mortality in cystic fibrosis patients.
- Medical device infections: Biofilms readily form on the surfaces of medical devices like catheters, cardiac implants, contact lenses etc.
- This can lead to life-threatening infections like ‘ESKAPE’ pathogens.
- Endocarditis: Biofilms formed by viridans group streptococci and Staphylococcus aureus on heart valves can cause infective endocarditis.
- Chronic inflammation and echocardiographic changes may necessitate valve replacement surgery.
- Otitis media: Middle ear infections caused by non-typeable Haemophilus influenzae and Streptococcus pneumoniae biofilms are persistent and resistant to antibiotics due to poor penetration.
- Chronic wounds: Biofilms formed by mixed species including Staphylococcus aureus, Pseudomonas aeruginosa and anaerobes impair the healing of chronic ulcers and diabetic foot infections. This can lead to limb amputation.

Diagnosis of Biofilms
Diagnosis of biofilm infections is challenging due to the lack of sensitive and specific tests. Some methods include:
- Microscopy: This involves the use of microscopes to visually inspect samples for the presence of biofilms.
- Techniques such as scanning electron microscopy (SEM) and confocal laser scanning microscopy (CLSM) can provide detailed images of biofilms and allow for the study of their structure.
- Culture: These techniques involve growing the microorganisms in a laboratory setting.
- Quantitative culture showing bacterial counts >103-104 CFU/g or mL from superficial swabs indicates biofilm.
- However, they are difficult to culture due to slow growth.
- Molecular techniques: These techniques involve the analysis of the genetic material of the microorganisms.
- Polymerase chain reaction (PCR) and other genetic analysis tools can be used to identify the specific types of microorganisms present.
- Antibiotic susceptibility: Increased antimicrobial tolerance is suggestive of a biofilm phenotype.
- Minimum inhibitory concentration determination can differentiate planktonic and biofilm bacteria.
- Biofilm reactor models: Experimental systems like the Calgary biofilm device allow the quantification of biofilms on various surfaces and test antibiotic susceptibilities. However, these are not used for clinical diagnosis.

Biofilms in Water Systems
| Issue | Control Strategy |
| Contamination and pathogens | – Disinfectants, UV, anti-fouling coatings |
| Corrosion and leaks | – Smooth pipe materials, corrosion inhibitors |
| Flow reduction | – Pigging, flushing, thermal shock |
| Regrowth prevention | – Alternating disinfectants, silver ionization |
Biofilms in Wastewater Treatment
They are an integral part of wastewater treatment systems:
| Role | Problem | Control |
| Degrade organics in filters and reactors | – Sloughing causes clogging | – Mechanical cleaning |
| Aid flocculation in activated sludge | – Filamentous bulking | – Reduce Food to Micro-organism Ratio (F: M) ratio |
| Perform membrane biodegradation | – Severe membrane biofouling | – Catalytic cleaners, disinfectant rotation |
| Produce methane in digesters | – Pipe/tank corrosion | – Aeration, mixing |
Biofilms in the Environment
Biofilms plays a significant role in the environment. They exist in various natural settings such as lakes, rivers, soil, rocks, aquatic plants, sediments, and wetlands.
- Water bodies: It grows on submerged rocks and sediments. They help purify water by metabolising nutrients, pollutants and pathogens. However, excess growth can clog water pipes and conduits.
- Soil: The rhizosphere around plant roots is coated with biofilms that protect plants, improve soil structure and promote mineralisation.
- Animals: Gut and skin microbiome biofilms in animals promote immunity and inhibit pathogens. Dental/intestinal biofilms also cause disease.
- Ships: Biofouling by marine bacteria and algae increases hydrodynamic drag. This reduces speed and fuel efficiency.
- Antifouling paints contain biocides to kill biofilms.
- Food industry: It helps produce fermented foods like cheese, yogurt and vinegar. But they also cause food spoilage and transmission of foodborne pathogens.
- Fossil fuels: Microbial biofilms contribute to microbial-enhanced oil recovery by increasing oil detachment and flow.
- But they also cause corrosion in oil reservoirs and pipelines.
- Bio-remediation: It helps purify contaminated wastewater and soils through biodegradation of pollutants. But can also clog remediation systems.
- Bio-corrosion: Sulfate-reducing bacteria biofilms corrode metals like iron, steel and concrete by generating hydrogen sulfide. This causes structural damage.
Thus, biofilms have diverse impacts on the environment. Careful control is needed to maximise beneficial effects while minimising the detrimental effects of biofilms in different environmental niches.
Last updated on April, 2025
→ UPSC Notification 2025 was released on 22nd January 2025.
→ The UPSC Vacancy 2025 were released 1129, out of which 979 were for UPSC CSE and remaining 150 are for UPSC IFoS.
→ UPSC Admit Card 2025 is expected to release in first week of May for CSE Prelims Exam 2025.
→ The UPSC Prelims 2025 is scheduled to be conducted on 25th May 2025 and UPSC Mains 2025 will be conducted on 22nd August 2025.
→ Apply once through it and aspirants can apply for various government exams conducted by UPSC.
→ The UPSC Selection Process is of 3 stages-Prelims, Mains and Interview.
→ UPSC Result 2024 is released with latest UPSC Marksheet 2024. Check Now!
→ UPSC Toppers List 2024 is released now. Shakti Dubey is UPSC AIR 1 2024 Topper.
→ Also check Best IAS Coaching in Delhi
Biofilms FAQs
Q1. How are biofilms formed?+
Q2. Why are biofilms resistant to antibiotics?+
Q3. What role do biofilms play in disease?+
Q4. How can biofilms be controlled?+