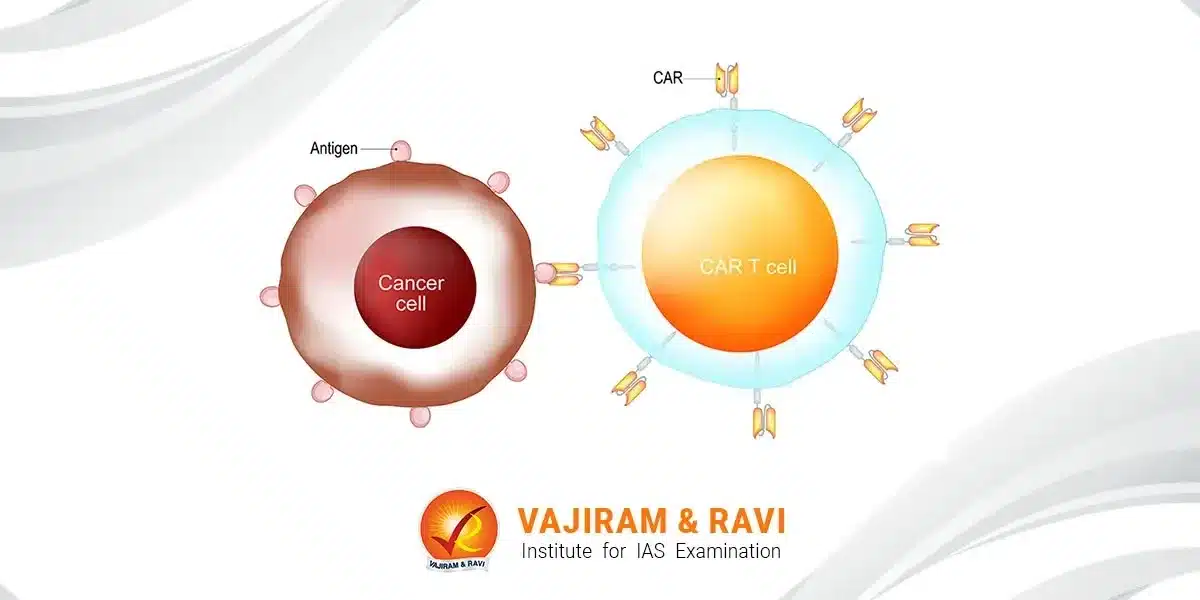
CAR T cell therapy is a promising new approach in cancer treatment that engineers a patient’s T cells to attack their tumours. It involves extracting T cells, genetically modifying them to target cancer antigens, multiplying them and reinfusing the engineered CAR T cells back into the patient’s body to provoke a potent immune response against cancer cells.
CAR T cell therapy has achieved remarkable outcomes in certain blood cancers, but limitations like toxicity risks and high costs remain. Understanding the mechanism, applications and future potential of this novel immunotherapy can provide insights into cutting-edge medical advances against cancer.
About CAR T Cell Therapy
CAR stands for chimeric antigen receptor. CAR T cell therapy involves collecting T cells from a patient’s blood and genetically engineering them to produce special receptors called chimeric antigen receptors (CARs) on their surface.
- These engineered CAR T cells can recognise and bind to specific protein targets (antigens) on cancer cells. The binding of the CAR T cells to cancer cells signals the T cells to become activated and multiply, and to kill the cancer cells.
- In contrast to general immunotherapies that boost the overall immune system, CAR T cell therapy involves engineering a patient’s immune cells to specifically attack their cancer.
- This makes the therapy a type of personalised cell therapy.
- CAR T cell therapy is being explored as a treatment option for different types of blood cancers including certain lymphomas and leukemias.
- The two CAR T cell therapies that are furthest along in development target the antigens CD19 and BCMA, which are found in B cells and plasma cells respectively.
Development of CAR T Cell Therapy
The concept of CAR T cell therapy has been in development since the late 1980s, but early clinical trials showed limited efficacy and safety.
- Advances over the last decade have significantly improved the design of CAR T cells and the clinical protocols, leading to improved safety and efficacy.
- U.S.A: Two CAR T cell therapies have now been approved by the US FDA – Kymriah in 2017 for B-cell acute lymphoblastic leukaemia, and Yescarta in 2017 for diffuse large B-cell lymphoma.
- India: The Central Drugs Standard Control Organisation (CDSCO), a drug regulatory body, approved NexCAR19 for commercial use in October 2023.
- Developed by ImmunoACT with Tata Memorial Hospital and IIT Bombay, it’s the first domestically made CAR-T therapy targeting B-cell cancers like leukaemia and lymphoma. It treats individuals above 15 with B-cell cancers.
- The therapy showcased its effectiveness with the remarkable recovery of the first patient. Accessible at a fraction of the cost compared to international alternatives, it emphasizes its affordability and potential to significantly impact patients’ lives.
- Many other CAR T cell therapies are currently in clinical trials for a range of cancers. Research is also exploring CAR T cell therapies for solid tumours, as well as “off-the-shelf” CAR T cell therapies using T cells from donors instead of the patient.
How CAR T Cell Therapy Works?
CAR T cell therapy is a type of immunotherapy that uses genetically modified immune cells to target and kill cancer cells. It is a personalised treatment that involves the following steps:
- T cell collection: Patient’s T cells are isolated from their blood using apheresis, a process similar to blood donation.
- T cell engineering: The collected T cells are genetically engineered to express the chimeric antigen receptor (CAR), using viruses as vectors. This gives the T cells specificity to target the cancer antigen.
- T cell expansion: The engineered CAR T cells are grown in the lab to expand their numbers to hundreds of millions. This process takes 2-3 weeks.
- Infusion into the patient: The expanded CAR T cells are infused back into the patient as an intravenous injection. The patient is usually pretreated with chemotherapy beforehand to deplete existing T cells.
- CAR T cells multiply and kill cancer cells: In the patient’s body, the infused CAR T cells bind to and kill cancer cells displaying the antigen on their surface. The CAR T cells proliferate and persist in the body, providing an ongoing cancer-killing ability.
Mechanism of Action
- The engineered CAR receptor on the T cells binds to the target antigen on cancer cells. This activates the T cell to proliferate and release cytotoxic proteins like perforin and granzymes, which enter the cancer cell and trigger cell death.
- CAR T cells also secrete cytokines like IL-2 and IFN-gamma that recruit and activate other immune cells to amplify the immune response against cancer cells across the body.
- CAR T cells can persist in the patient for years after infusion, providing durable anti-cancer activity. However, some patients relapse if their cancer stops expressing the target antigen.
CAR T Cell Generations
- First-generation: CARs only had the CD3-zeta signalling domain, providing suboptimal activation of T cells.
- Second-generation: CARs added a costimulatory domain (e.g. CD28, 4-1BB), improving T cell activation and persistence.
- Third-generation: CARs contain two costimulatory domains for greater T cell activity but increased toxicity risk.
- Fourth-generation: CARs additionally express cytokines or other enhancements to improve efficacy and safety.
Types of CAR T Cell Therapies
While most approved CAR T cells target CD19 in B cell leukaemias and lymphomas, new targets are expanding applications:
- CD19 CAR T Cells: CD19 is a cell surface marker on B cells and B cell cancers.
- These cells have induced high remission rates in refractory B cell acute lymphoblastic leukaemia (ALL) and certain lymphomas.
- BCMA CAR T Cells: B cell maturation antigen (BCMA) is expressed on multiple myeloma cells.
- These cells have achieved robust response rates in relapsed/refractory myeloma.
- Other CAR Types: CARs targeting antigens like CD20, CD22 and CD30 are also being evaluated for hematologic malignancies.
- CAR T cells targeting solid tumour antigens(e.g. MUC1) are in early-phase trials.
Toxicities and Side Effects
CAR T cell therapy has achieved remarkable outcomes in certain blood cancers, but toxicity risks and Side effects remain as follows:
- Cytokine release syndrome (CRS): Caused by high levels of inflammatory cytokines released by activated CAR T cells. Symptoms may include fever, low blood pressure, difficulty breathing and organ toxicities. CRS can be severe or life-threatening in some cases.
- Neurological toxicity: Some patients experience neurological issues like delirium, seizures or cerebral oedema, likely also related to cytokine elevations. These are usually reversible.
- On-target off-tumor toxicity: If the target antigen is also expressed on some normal cells, the CAR T cells may cause toxicity to those tissues.
- For example, CD19 CAR T cells can deplete normal B cells.
- Other side effects can include infections, anaemia, neutropenia and hypogammaglobulinemia. Supportive care measures are given to manage toxicities.
- Risk mitigation strategies for CAR T cell toxicity include dose adjustment, incorporating safety switches, and cytokine blockade.
Current Challenges and Limitations
Some challenges and limitations need to be overcome before CAR T-cell therapy can be widely applied to solid tumours and haematological malignancies.
- Relapses can occur in some patients due to antigen escape when cancer cells stop expressing the CAR’s target antigen. Combination treatments or CAR T cells targeting multiple antigens may help prevent relapse.
- Efficiency: CAR T cell therapies have mostly been successful against blood cancers, with limited efficacy seen so far against solid tumours. Additional bioengineering strategies are being explored to improve solid tumour treatments.
- Toxicity: Severe toxicities like cytokine release syndrome and neurological issues can occur in some patients after CAR T cell infusion, although usually manageable.
- Complex, personalised manufacturing is logistically challenging. Allogeneic off-the-shelf CAR T cell products from donors aim to simplify manufacturing and administration.
- High costs and reimbursement difficulties make CAR T cell therapies inaccessible for many patients. Health systems need funding models to improve access.
Way Forward
CAR T cell therapy is currently very expensive with treatment costing several hundred thousand dollars per patient. The future outlook remains promising – CAR T cell research is surging ahead to:
- CAR T cell therapy is poised to become a major advancement in cancer treatment alongside surgery, chemotherapy and radiation.
- Ongoing innovation is expanding the range of cancer targets and working to improve efficacy, safety and accessibility.
- It may eventually be an off-the-shelf treatment used earlier in cancer therapy, rather than later.
- Combination regimens with checkpoint inhibitors, bispecific antibodies or systemic immunomodulators may further enhance CAR T cell potency.
- Next-generation enhancements like TRUCKs, ARM T cells and the use of iPSC-derived NK or T cells may also help overcome current limitations.
- CAR T cell therapy is on the leading edge of personalised cell therapies and cancer immunotherapy advances.
It is thus spearheading innovation at the confluence of cell engineering, gene therapy and synthetic biology. The decades ahead will see continuous refinements potentiate CAR T cells into a transformative anti-cancer platform technology.
Last updated on June, 2025
→ UPSC Notification 2025 was released on 22nd January 2025.
→ UPSC Prelims Result 2025 is out now for the CSE held on 25 May 2025.
→ UPSC Prelims Question Paper 2025 and Unofficial Prelims Answer Key 2025 are available now.
→ UPSC Calendar 2026 is released on 15th May, 2025.
→ The UPSC Vacancy 2025 were released 1129, out of which 979 were for UPSC CSE and remaining 150 are for UPSC IFoS.
→ UPSC Mains 2025 will be conducted on 22nd August 2025.
→ UPSC Prelims 2026 will be conducted on 24th May, 2026 & UPSC Mains 2026 will be conducted on 21st August 2026.
→ The UPSC Selection Process is of 3 stages-Prelims, Mains and Interview.
→ UPSC Result 2024 is released with latest UPSC Marksheet 2024. Check Now!
→ UPSC Toppers List 2024 is released now. Shakti Dubey is UPSC AIR 1 2024 Topper.
→ Also check Best IAS Coaching in Delhi
Car T Cell Therapy FAQs
Q1. What are CAR T cells?+
Q2. How are CAR T cells administered?+
Q3. What types of cancer is CAR T cell therapy used for?+
Q4. What are the side effects of CAR T cell therapy?+
Q5. How much does CAR T cell therapy cost?+
Tags: car t cell therapy quest