What’s in today’s article?
- Why in News?
- What is Gene Therapy?
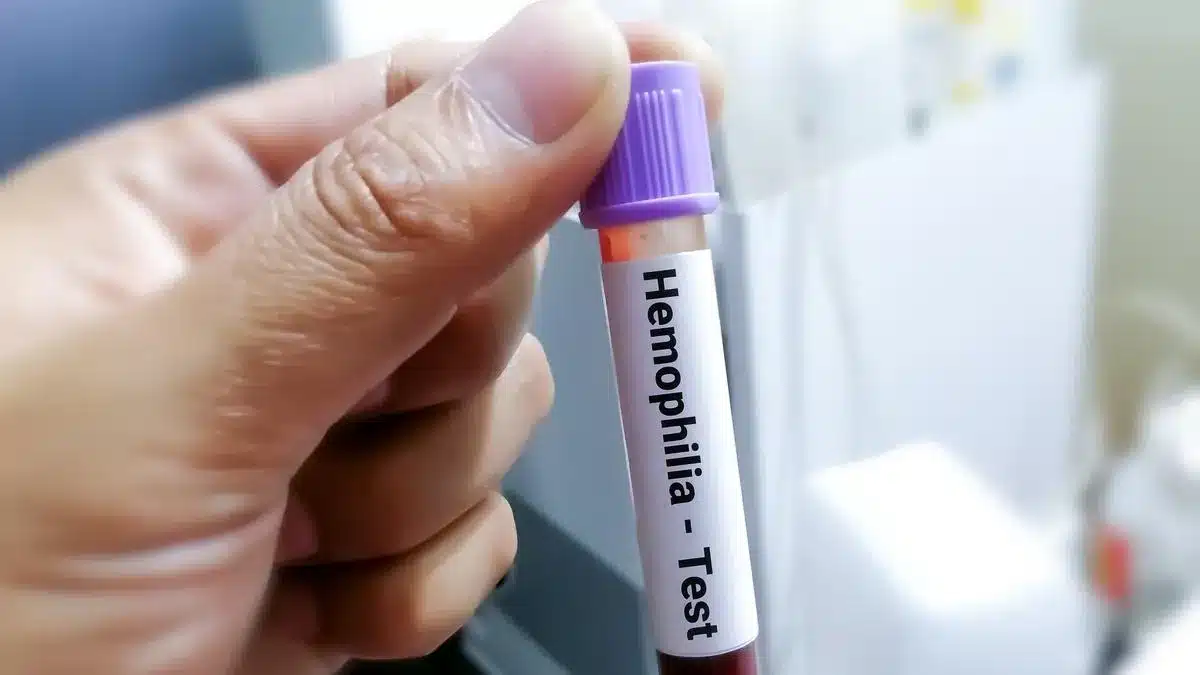
- Understanding Hemophilia A
- The Promise of Gene Therapy
- Global Context of Gene Therapy
- Significance of the Gene Therapy Success in India
- Conclusion
Why in News?
- Indian scientists have achieved a major milestone by using gene therapy to treat severe Hemophilia A, a rare genetic condition causing life-threatening bleeding episodes.
- This pioneering work, tested on five patients in Tamil Nadu, has shown promising results, with no bleeding episodes reported over an average follow-up period of 14 months.
What is Gene Therapy?
- Human gene therapy seeks to modify or manipulate the expression of a gene or to alter the biological properties of living cells for therapeutic use.
- It is used to treat or cure disease including cancer, genetic diseases, and infectious diseases.
- Gene therapies can work by several mechanisms:
- Replacing a disease-causing gene with a healthy copy of the gene.
- Inactivating a disease-causing gene that is not functioning properly.
- Introducing a new or modified gene into the body to help treat a disease.
- There are a variety of types of gene therapy products, including:
- Plasmid DNA: Circular DNA molecules can be genetically engineered to carry therapeutic genes into human cells.
- Viral vectors: Once viruses have been modified to remove their ability to cause infectious disease, these modified viruses can be used as vectors (vehicles) to carry therapeutic genes into human cells.
- Bacterial vectors: Bacteria can be modified to prevent them from causing infectious disease and then used as vectors (vehicles) to carry therapeutic genes into human tissues.
- Human gene editing technology: The goals of gene editing are to disrupt harmful genes or to repair mutated genes.
- Patient-derived cellular gene therapy products: Cells are removed from the patient, genetically modified (often using a viral vector) and then returned to the patient.
Understanding Hemophilia A:
- What is Hemophilia?
- Hemophilia is a rare, inherited blood disorder that prevents blood from clotting properly.
- Hemophilia can be classified as minor or severe depending on the percentage of clotting factors present in those afflicted.
- What is Hemophilia A?
- Meaning: It is a rare hereditary disorder caused by the absence of Factor VIII, a critical blood-clotting protein.
- Severity: Classified as minor or severe based on clotting factor levels; severe cases have less than 1% clotting factor.
- Global context: India has the world’s second-largest patient pool, estimated at 40,000-100,000.
- Current treatments:
- Frequent interventions: Repeated Factor VIII infusions, monoclonal antibodies, or mimicking substances are used.
- High costs: Treatment costs in India are approximately ₹2.54 crore per patient over 10 years, making it inaccessible for many.
The Promise of Gene Therapy:
- How does it work?
- One-time solution: Gene therapy introduces a functional gene that enables the body to produce sufficient Factor VIII, reducing or eliminating the need for repeated infusions.
- Innovative technique used: The Indian trial fused stem cells with the clotting factor gene using lentivirus (a safer vector compared to adenovirus), which eliminates the need for immunosuppressive drugs.
- Results from the trial:
- Patients: Five individuals treated, with no bleeding episodes over 14 months.
- Research team: Led by Alok Srivastava from the Christian Medical College (CMC), Vellore.
- Support: Funded by the Union Department of Biotechnology.
Global Context of Gene Therapy:
- Roctavian: Approved by the U.S. FDA in 2023, reducing bleeding incidents significantly in patients.
- Mechanism: Uses adenovirus vectors to deliver the therapeutic gene, requiring immune suppression.
Significance of the Gene Therapy Success in India:
- Experts called the study “ground-breaking” due to:
- Resource constraints: Demonstrating the feasibility of conducting advanced gene therapy in a developing country.
- Cost reduction: Potential for localising gene therapy manufacturing in India, improving accessibility.
- Broader access: Overcoming barriers like immunosuppressive therapy and age limitations. This method may allow younger patients to receive treatment, overcoming challenges like liver maturity and health.
Conclusion:
- The success of this gene therapy trial in India represents a transformative step in treating Hemophilia A, offering a safer, more accessible, and effective solution.
- This breakthrough not only holds promise for India but also sets a global precedent for advancing medical care in resource-constrained settings.
Q.1. What is CRISPR-Cas9?
CRISPR-Cas9 is a unique technology that enables geneticists and medical researchers to edit parts of the genome by removing, adding or altering sections of the DNA sequence.
Q.2. What is the Human Genome Project (HGP)?
The HGP was an international scientific research project with the goal of determining the base pairs that make up human DNA, and of identifying, mapping and sequencing all of the genes of the human genome from both a physical and a functional standpoint. It started in 1990 and was completed in 2003.
News: Indian scientists develop novel gene therapy treatment for haemophilia | BS
Last updated on June, 2025
→ UPSC Notification 2025 was released on 22nd January 2025.
→ UPSC Prelims Result 2025 is out now for the CSE held on 25 May 2025.
→ UPSC Prelims Question Paper 2025 and Unofficial Prelims Answer Key 2025 are available now.
→ UPSC Calendar 2026 is released on 15th May, 2025.
→ The UPSC Vacancy 2025 were released 1129, out of which 979 were for UPSC CSE and remaining 150 are for UPSC IFoS.
→ UPSC Mains 2025 will be conducted on 22nd August 2025.
→ UPSC Prelims 2026 will be conducted on 24th May, 2026 & UPSC Mains 2026 will be conducted on 21st August 2026.
→ The UPSC Selection Process is of 3 stages-Prelims, Mains and Interview.
→ UPSC Result 2024 is released with latest UPSC Marksheet 2024. Check Now!
→ UPSC Toppers List 2024 is released now. Shakti Dubey is UPSC AIR 1 2024 Topper.
→ Also check Best IAS Coaching in Delhi