About Fuel Cell
- A fuel cell is a device that generates electricity by a chemical reaction.
- Fuel cells can be used in a wide range of applications, providing power for applications across multiple sectors, including transportation, industrial/commercial/residential buildings, and long-term energy storage for the grid in reversible systems.
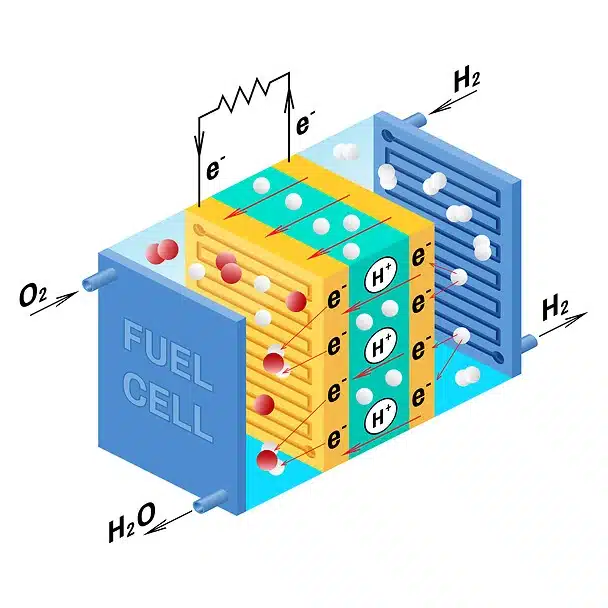
- Working:
- A fuel cell consists of two electrodes—a negative electrode (or anode) and a positive electrode (or cathode).
- Both electrodes must be immersed in and separated by an electrolyte, which may be a liquid or a solid but must, in either case, conduct ions between the electrodes in order to complete the chemistry of the system.
- A fuel, such as hydrogen, is supplied to the anode, where it is oxidized, producing hydrogen ions and electrons.
- An oxidizer, such as oxygen, is supplied to the cathode, where the hydrogen ions from the anode absorb electrons from the latter and react with the oxygen to produce water.
- The difference between the respective energy levels at the electrodes (electromotive force) is the voltage per unit cell.
- The amount of electric current available to the external circuit depends on the chemical activity and amount of the substances supplied as fuel.
- A single fuel cell generates a tiny amount of direct-current (DC) electricity. In practice, many fuel cells are usually assembled into a stack.
- Advantages of Fuel Cells:
- Fuel cells have lower or zero emissions compared to combustion engines. Hydrogen fuel cells emit only water, addressing critical climate challenges as there are no carbon dioxide emissions.
- There are also no air pollutants that create smog and cause health problems during the operation of a fuel cell.
- They are quiet during operation as they have few moving parts.
- They can operate at higher efficiencies than combustion engines.
- A fuel cell resembles a battery in many respects, but it can supply electrical energy over a much longer period of time.
- This is because a fuel cell is continuously supplied with fuel and air (or oxygen) from an external source, whereas a battery contains only a limited amount of fuel material and oxidant that are depleted with use.
Q1) What are electrodes?
An electrode is a conductor that is used to make contact with a nonmetallic part of a circuit. Electrodes are commonly used in electrochemical cells (see Figure 1), semiconductors like diodes, and in medical devices. The electrode is the place where electron transfer occurs.
Source: Isro tests futurist fuel cell system that could power space station
Last updated on July, 2025
→ UPSC Notification 2025 was released on 22nd January 2025.
→ UPSC Prelims Result 2025 is out now for the CSE held on 25 May 2025.
→ UPSC Prelims Question Paper 2025 and Unofficial Prelims Answer Key 2025 are available now.
→ UPSC Calendar 2026 is released on 15th May, 2025.
→ The UPSC Vacancy 2025 were released 1129, out of which 979 were for UPSC CSE and remaining 150 are for UPSC IFoS.
→ UPSC Mains 2025 will be conducted on 22nd August 2025.
→ UPSC Prelims 2026 will be conducted on 24th May, 2026 & UPSC Mains 2026 will be conducted on 21st August 2026.
→ The UPSC Selection Process is of 3 stages-Prelims, Mains and Interview.
→ UPSC Result 2024 is released with latest UPSC Marksheet 2024. Check Now!
→ UPSC Toppers List 2024 is released now. Shakti Dubey is UPSC AIR 1 2024 Topper.
→ Also check Best IAS Coaching in Delhi